CAR-T cell therapy has changed how we treat fast-growing blood cancers, like leukaemia and large B-cell lymphoma. It works modifying a patient’s own T cells so they can target and destroy cancer cells. The next steps developers is in vivo CAR-T. Put simply, this method engineers the T cells inside the body rather than in a lab. This cuts waiting times , lower costs, and greater would make treatment more available to more people. However, it also increases the need for precise vector design and strict quality controls.
Current CAR-T Therapy Available
Currently, seven CAR-T therapies approved by the FDA. Four target CD19 to treat B-cell leukaemia and lymphoma, and two target BCMA for difficult to treat myeloma.
Most success so far has been in these blood cancers, but because CAR molecules can be redesigned, they are being tested in many other areas.
New versions are being developed for solid tumours, autoimmune diseases and even some infections. After a decade of strong clinical results, CAR-T has proven itself as a powerful new way to treat disease.
The Shift Toward In Vivo CAR-T
A major advance in CAR-T therapy is the move toward in vivo CAR-T.
Instead of modifying T cells in a lab, this approach uses viral vectors or lipid nanoparticles to engineer them inside the body.
This may shorten manufacturing time, reduce costs, and make treatment more widely available — with the potential for fewer side effects.
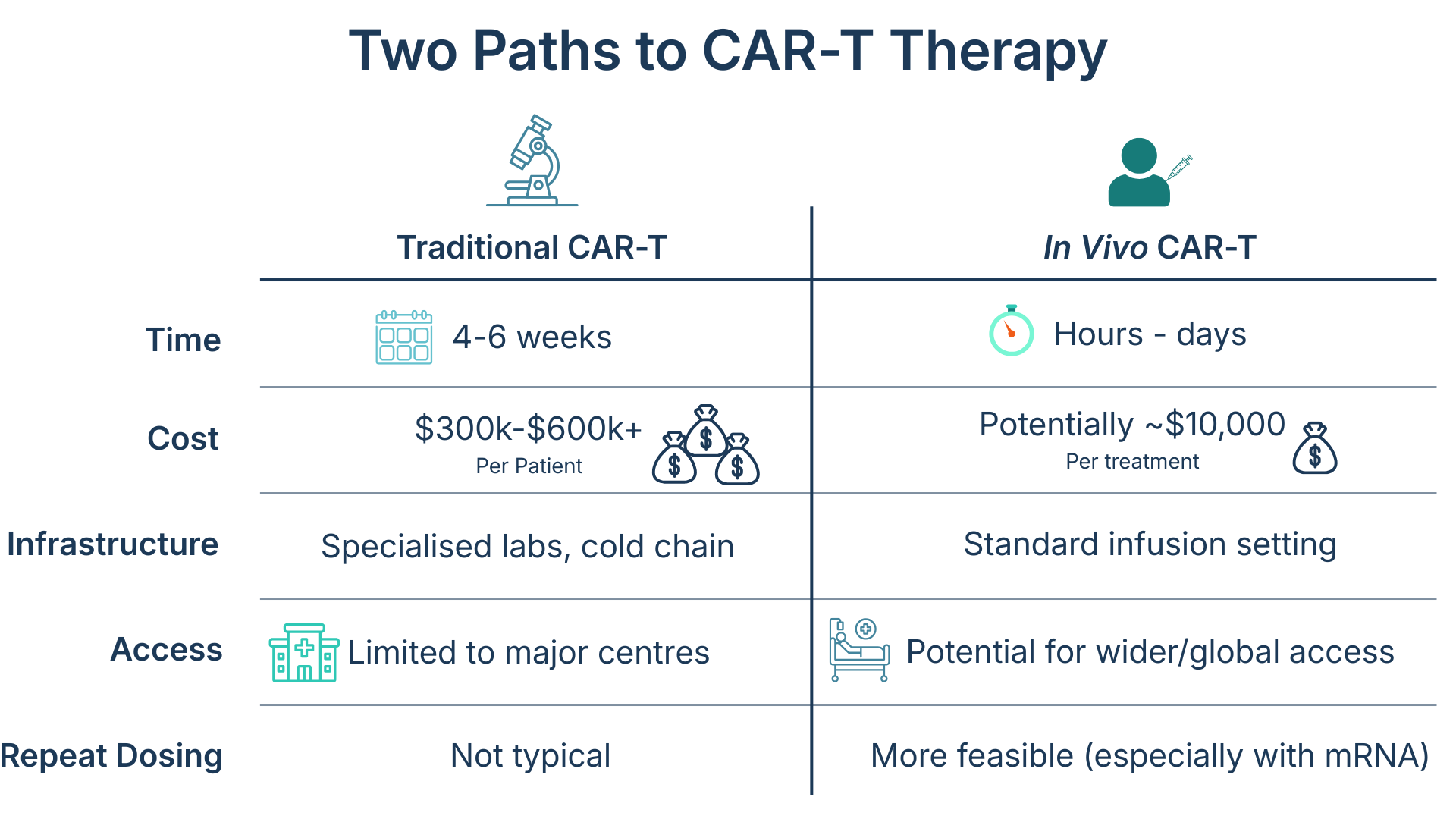
Traditional CAR-T vs. In Vivo CAR-T
Traditional CAR-T manufacturing involves:
- Collecting T cells
- Modifying them ex vivo using viral vectors or genetic engineering tools
- Growing them
- Infusing them back into the patient
This takes 4–6 weeks and requires specialized facilities,which limits access to major treatment centres.
In vivo CAR-T, replaces this whole process. A viral vector or lipid nanoparticle is injected directly into the patient delivering the CAR gene straight to their T-cells.
The cells then modify and grow inside the body, removing the need for lab processing and cutting treatment time dramatically.
Advantages and Challenges of In Vivo CAR-T
In vivo CAR-T come with several benefits. It may remove the need for pre-treatment chemotherapy, cut waiting times, lower production costs and enable scaling from a single vector batch. It could also open doors to treating solid tumours, autoimmune disorders, and fibrotic diseases.
However, direct in vivo delivery raises key challenges:
- Vectors must reach the T cell efficiently
- The vector must be pure, or contaminants may trigger immune reactions
- Dosing is harder to control without lab oversight
- The immune system may react to vectors or CAR protein, making repeat dosing difficult.
Current Developments and Clinical Trials
In vivo CAR-T can produce CAR-T cells within hours instead of months. This represents a major improvement over traditional manufacturing.
Researchers are testing viral and non-viral delivery methods, including lentiviral vectors and lipid nanoparticles.
Several companies are already running early clinical studies. Capstan Therapeutics is testing lipid nanoparticles (LNPs) that deliver mRNA to T cells. Umoja Biopharma is using lentiviral vectors in trials for B cell lymphoma. The goal is to match effectiveness of today’s CAR-T therapies while making treatment faster and simplifying delivery. With these programmes moving forward, one challenge becomes increasingly important: vector purity. Ensuring vector quality becomes even more critical when therapies are delivered directly into patients
Why Vector Purity Matters More for In Vivo CAR-T
In in vivo CAR-T, the viral vector is injected directly into the patient. So any leftover impurities — like host-cell DNA or proteins — also go straight into the body.
Because there is no extra processing after dosing, the vector itself must meet the highest purity standards from the start.
Ex vivo CAR-T works differently. Here, the vector only modifies T cells inside a controlled lab. After transduction, the cells go through wash steps and days of expansion. These stages naturally remove most vector-related contaminants long before the final CAR-T product is reinfused. In other words, the lab process acts like a built-in purification system, reducing impurities by several orders of magnitude.
This fundamental difference is why purity becomes such a priority in in vivo CAR-T. With no downstream clean-up steps to rely on, the vector must be clean at the point of manufacture — making DNA removal essential for safe and scalable in vivo therapy development.
Ensuring Vector Purity
One of the biggest challenges in in vivo CAR-T manufacturing is producing a vector that meets the strict purity standards required for direct administration into patients. Traditional nucleases can struggle to digest chromatin-bound DNA, which may lead manufacturers to introduce additional digestion steps or higher enzyme amounts to reach the needed level of clearance.
This can introduce two kinds of risk:
• Residual host-cell DNA, which may trigger immune reactions or raise regulatory concerns
• Carryover nuclease, which becomes more likely when multiple digestion steps or large enzyme quantities are used
Because M-SAN HQ GMP and SAN HQ GMP maintain strong activity under the salt conditions commonly used in lentiviral and gene-therapy workflows, they can achieve effective DNA digestion in a single step and at lower enzyme concentrations. This reduces the need for repeated treatments and therefore lowers the chance of nuclease carryover in the final product.
This simplified approach supports more consistent impurity removal, reduces the likelihood of aggregation, and results in a cleaner vector that aligns with purity expectations for in vivo use. For manufacturers working at physiological salt levels, M-SAN HQ GMP is specifically designed for these conditions. For high-salt workflows, SAN HQ GMP provides the same advantages under elevated-salt conditions.
Together, these enzymes support more streamlined downstream processing and contribute to safer, more reliable vectors for in vivo CAR-T therapies.
Summary
In vivo CAR-T could reshape cancer treatment by letting the body make its own CAR T cells. This removes many manufacturing barriers and could give more patients access to therapy.
Challenges remain — including immune reactions and vector purity — but better delivery and purification tools are helping move the field forward.
With several clinical trials underway, in vivo CAR-T is moving closer to treating blood cancers, autoimmune disease, and possibly solid tumours.
At ArcticZymes, we support this progress with nucleases that help improve vector purity and safety. Learn more about our novel enzymes here.